Australian Parkinson’s Mission Update
Sunday, 23rd August 2020
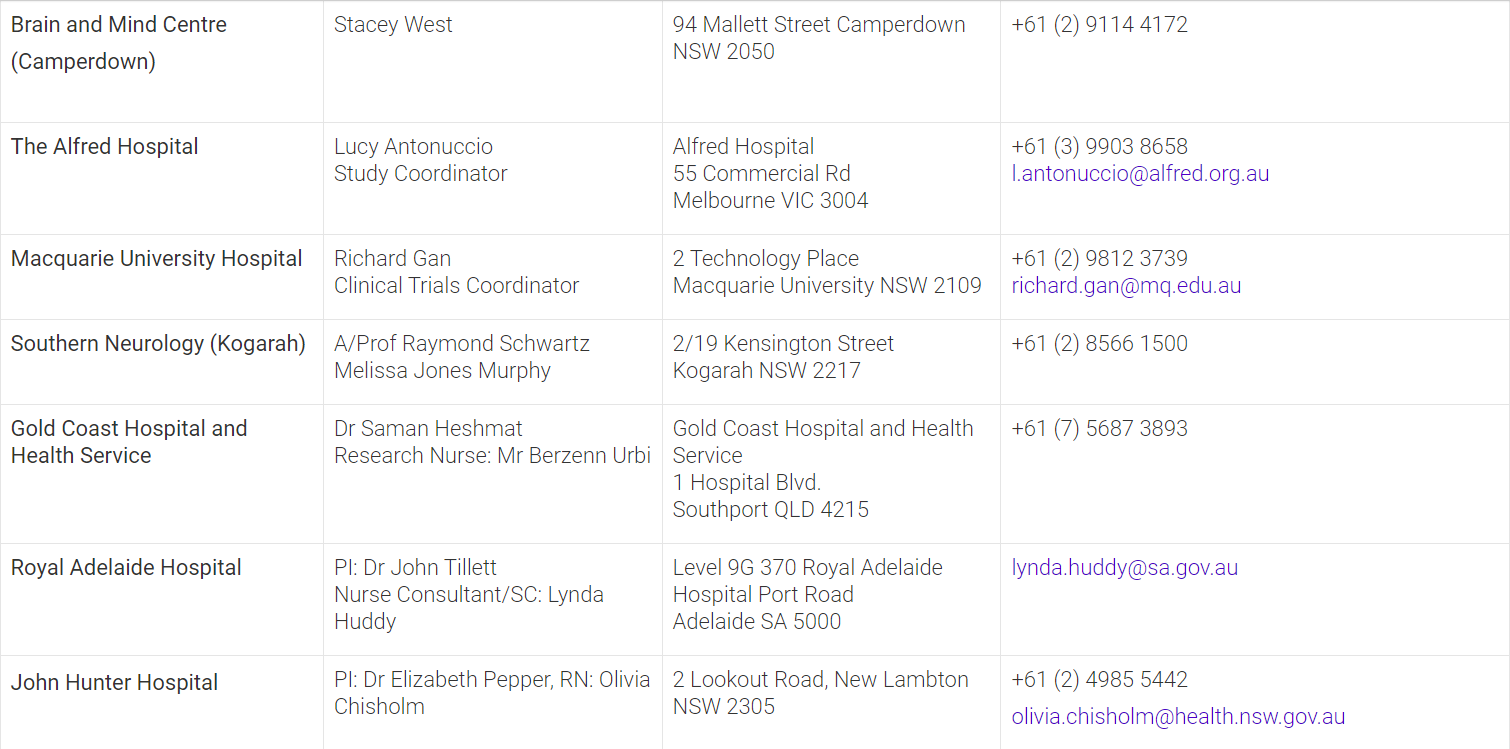
The mission of the APM is to conduct innovative clinical trials integrated with genomic and biomarker approaches to revolutionise our understanding of Parkinson’s disease and find treatments to slow and stop the disease progression. To determine whether you are eligible to participate in the APM001 clinical trial, please contact the closest site in your state.
Clinical Trial Site Locations
For more information visit:
theapm.org.au
Email: info@TheAPM.org.au